This page is limited to our registered members
-
Get access to exclusive dermatological services to increase your professionnal knowledge: +500 pathology visuals, clinical cases, expert videos
-
Benefit from valuable features: audio listening, materials to be shared with your patients
-
Stay informed about the upcoming events and webinars, latest scientific publications and product innovations
Already have an account? login now
BIODERMA Congress Reports ESPD 2022
Reports written by Dr. Nives Pustisek (Pediatric dermatologist, Croatia), Prof. Ivelina Yordanova (Dermatologist, Bulgaria) and Dr. Rémi Maghia (Dermatologist, France)
Related topics
Dear colleagues,
It was my pleasure and honor to participate in the first ESPD meeting in person since the pandemic. We have all accepted that different video platforms are serving the purpose, however contact in person and live discussions are still the top choice when talking about efficient transfer of knowledge and deepening the relationship among colleagues professionals. Having said that, let me compliment an excellent scientific program and opportunity to socialize with colleagues during the ESPD meeting in Munich this year. As you are aware, the program went on in two parallel sessions. Please find below several reviews of very interesting lectures.
Speakers: Prof. Antonio Torrelo, Prof. Milos Nikolic and Prof. Henning Hamm
Report written by Dr. Nives Pustisek
Skin phenotypes in autoinflammation
Prof. Antonio Torrelo (Spain)
The lecture began with the classification of autoinflammatory diseases (Dilan Dissanyake, SPD meeting 2021, Toronto): inflammasomopathy, interferonopathy, NF-kBopathy. Each group shared some clinical characteristics, variable phenotypes and treatment options. Inflammasomopathy are a heterogeneous group of genetic diseases, with variable cytokine profiles (IL-1ß, IL-18, IL-36) and they are called IL-1 driven autoinflammatory diseases. Clinical characteristics of inflamasomopathy include fevers, organ involvement (abdominal pain, non-vasculitis rashes, uveitis, arthritis), elevated WBC/neutrophils, highly elevated inflammatory markers. This group is associated with variable phenotypes (urticarial, edematous, pustular, neutrophilic, psoriasiform and others) and variable systemic signs. Treatment includes IL-1 blockade (anakinra, rilonacept, canakinumab), IL-18 blockade (tadekinig-alfa-IL-18BP), IL-36 (spesolimab). Vey interesting case reports have been presented, such as Criopyrin associated periodic syndromes (CAPS; NLRP3 pathogenic change identified) and Deficiency of IL-36 receptor antagonist (DITRA). Clinical characteristics of interferonopathy include fevers, organ involvement (vasculitic rashes, interstitial lung disease, intracranial calcifications), inflammatory markers may not be as elevated, autoantibodies may be present. They are treated by JAK inhibitors. In this group of patients, as well, interesting cases have been presented and a list of major monogenic interferonopathy and skin features. Clinical characteristics of NF-kBopathy include fevers, highly variable organ involvement (oral/GI/GU ulceration, granulomas).
In conclusion, we can suspect an autoinflammatory disease when we are presented with a non- infectious periodic fever and skin lesions; skin lesions triggered by common triggers; early onset connective tissue disease; recurrent panniculitis in a young child; cutaneous vasculitis and systemic signs; unexpected skin lesions and systemic signs; inflammatory skin disease with minimal lab anomalies; severe, intractable disorder of keratinization; skin lesions of infancy with predominant neutrophils; unusual skin lesions and immunodeficiency and cytopenias. Additionally, another sign that the disease is of autoinflammatory nature is when we’re not sure what it is. Around 50 well- characterized autoinflammatory disease exist. But still, only around 50% of patients with autoinflammatory diseases have a gene.
Juvenile dermatomyositis: what's new?
Prof. Milos Nikolic (Serbia)
Juvenile dermatomyositis (JDM) is a rare, serious systemic autoimmune disease, immune occlusive small-vessel vasculopathy. It’s the most common juvenile idiopathic inflammatory myopathy that affects skin, skeletal muscle, joints, gastrointestinal tract, heart and lungs. Some patients (1-5%) have amyopathic JDM (of these 25% developed overt JDM). 1/3 patients have acute, monocyclic course (up to 2 years); ¼ have polycyclic disease. More than 50% have chronic, continuous disease despite treatment. Even after 16 years of evolution, more then 50% of JDM patients still have an active disease (capillary nailfold changes, skin rash and others). Before the steroid era, 1/3 patients died, 1/3 had significant disability and 1/3 completely recovered. Today the mortality rate is more then 2%. JDM is generally not paraneoplastic. Very rare paraneoplastic cases have been presented in the literature. The incidence is 1-4 / 1 000 000 children/year, girls:boys 3:1. Etiopathogenesis is complex, immunological dysfunction (genetic background) and environmental stimuli / triggers (infection, drugs, UV light etc.). Some antibodies have the same association as in adults: anti-PM-Scl (overlap with scleroderma), anti-Mi-2 (classic skin rash, milder muscle involvement, lower risk of interstitial pneumonia, respond well to therapy), ant-Jo-1 (interstitial lung disease). Some antibodies are associated with a mild form of disease like anti-MDAS and anti-SAE. Anti-Ro (SSA) are associated with poor prognosis. The new Classification criteria 2017 for adult and juvenile inflammatory myopathies has been presented. The second part of the presentation included patients with JDM from the Department of Dermatology, Belgrade, 1990-2021, clinical presentation, disease course and the complexity of treatment options.
Psoriasis
Prof. Henning Hamm (Germany)
Pediatric psoriasis is a systemic inflammatory immune-mediated disease. 30% of patients have disease onset before the age of 20 years. Prevalence in the European children and adolescents 0.5- 1%. Pediatric psoriasis shows a strong genetic predisposition, most important PSORS1, HLA-Cw6. Pathogenesis is very complex, stimulation of keratinocytes by proinflammatory cytokines (T cells: IL- 17A, IL 6, IL8, TNFα; dendritic cells: IL-1ß, IL-12, IL-23, THFα). Pediatric psoriasis is associated with a number of comorbidities (metabolic, cardiovascular, articular, gastrointestinal and psychological, most important obesity). It has a strong influence of patient quality of life and also parents and family quality of life. Indication for systemic treatment, moderate to severe psoriasis defined by Psoriasis Area and Severity Index (PASI) ≥10, Body Surface Area (BSA) ≥10, Children’s Dermatology Life Quality Index (CDLQI) or Dermatology Life Quality Index (DLQI) ≥16 years ≥10; predominant involvement of sensitive sites (scalp, face, palms, soles, nails, genital and intertriginous areas) not sufficiently considered in PASI and BSA. Five biologic agents have been approved for pediatric psoriasis (etanercept, adalimumab, ustekinumab, ixekinumab, scukunumab). The German guidelines update 2021 and diagnostic criteria for pediatric psoriasis have also been presented. The clinical presentation and treatment for Generalized pustular psoriasis annular type has also been presented. “Psoriasis dermatitis” is an overlap condition of psoriasis and atopic dermatitis. Psoriasiform dermatitis during dupilumab treatment and paradoxal psoriasis (psoriasifom lesions induced by biologics, mainly TNF-alfa inhibitors) have been presented.
Speakers: Prof. May El Hachem, Prof. Stephanie Christen-Zäch and Prof. Antonella Muraro
Report written by Dr. Nives Pustisek
Photosensitivity in children
Prof. May El Hachem (Italy)
Photosensitivity is an abnormal response to sun exposure or to an artificial light source, with variable skin eruption manifestations and pruritus. The presentation included a classification of photosensitivity: primary photosensitivity (Polymorphous light eruption, Actinic pruritus, Juvenile spring eruption, Solar urticaria, Hydroa vacciniforme), due to exogenous agents (Drug induced, Phytophotodermatitis), connective tissue diseases (Neonatal lupus erythematosus, Systemic lupus erythematosus, Juvenile dermatomyositis), photoaggravated dermatoses (Herpes simples, Psoriasis, Atopic dermatitis, lichen, Darier’s disease), different metabolic disorders (Erythropoetic protoporphyria, Congenital erythropoetic porphyria and others) and genodermatoses (Trichothiodystropy, Xeroderma pigmentosum, Rothmund-Thomson syndrome, Kindler EB and others). Through interesting case reports different photosensitivity disorders have been presented with differential diagnoses, diagnostic and therapy. The diagnostic work up includes clinical and family history (date of onset of the lesions, relationship with sun exposure, evolution, drug assumption or use of topical product, associated disorders, family history (genodermatoses, connective diseases, etc.), clinical exam, laboratory tests (depending on the clinical diagnostic hypothesis), phototesting (in selected cases), skin biopsy and other investigations.
In conclusion, photosensitivity is present in several disorders, it may exacerbate multiple common diseases. Accurate clinical history and exam should be performed. Early diagnosis is required to avoid complications and to guarantee an appropriate therapy, differential diagnosis should be always considered. Diagnostic management depends on the clinical diagnostic hypothesis. Multidisciplinary management is frequently necessary.
Update on chronic urticaria in children
Prof. Stephanie Christen-Zäch (Switzerland)
Etiology of urticaria and angioedema: infections, allergic (food, drugs, contact allergens, aeroallergens), pseudo-allergic (aspirin, NSAIs, food additives, local anesthesia, radiocontrast agents), physical (mechanic, light, cold/heat), toxic (insects, plants, jellyfish, chemical substances), cholinergic (when exercising), endogenic (auto immune, auto-inflammatory, hematologic) and idiopathic. If one is presented with an angioedema without urticaria, this could point to C1 esterase inhibitor deficiency, angiotensine-converting enzyme inhibitors, hereditary angioedema (bradikinin mediated). Clinical presentation of urticaria can be in the classical form, figurated urticaria, dermographismus, urticarial papules (cholinergic urticaria), giant urticaria (in babies, appearance like a bruise, frequent location on hands and feet). Urticaria vasculitis, Serum sickness reactions, Acute hemorrhagic edema of infancy, Maculopapular, cutaneous mastocytosis, Auto-inflammatory syndromes, Nonmast cell mediator-mediated angioedema can be manifested with wheals or angioedema, but not considered to be subtypes of urticaria as they have different pathophysiologic mechanisms. Urticaria classification: acute urticaria (less than 6 weeks), chronic urticaria (more than 6 weeks), spontaneous urticaria (no specific eliciting factor involved) and inducible urticaria (specific eliciting factor involved). Chronic urticaria subtypes can be chronic spontaneous urticaria (CSU) and chronic inducible urticaria (physical or other). Duration of CSU is estimated to be 1-5 years in most cases. In diagnostic work up of urticaria one should not do intensive and costly general screening program, not all possible causative factors need to be investigated in all patients. The most important thing is patient’s history, physical examination and in some patients when is indicated further appropriate diagnostic tests (look in diagnostic algorithm for chronic urticaria (Allergy 2018). In the final part of the lecture, the treatment algorithm for urticaria according EAACI/GA2LEN/EDF/WAO guidelines (Allergy, 2018) and strong influence of chronic urticaria on quality of life have been presented.
Food Allergies
Prof. Antonella Muraro (Italy)
The lecture exhibited a connection between food allergy and atopic dermatitis (an in-parallel journey?), pathophysiology and the link between food allergy and atopic dermatitis: epicutaneous sensitization, and diagnostic work-up of food allergy in atopic dermatitis and prevention strategies. Food allergies and atopic dermatitis appear to be closely associated, both represent a public health burden with a huge psychosocial impact on children and their families. In children below 6 years of age with mild atopic dermatitis, a diagnostic work up for food allergy may be warranted if the history is positive for immediate reaction to food. In moderate atopic dermatitis if there is a poor response to an adequate local treatment or positive history for immediate reaction to food. Introduction of egg and peanut early between 4 and 6 months of age is suggested to prevent food allergy especially in high risk children with mild atopic dermatitis and in countries with high prevalence of peanut allergy. Optimizing the care of the skin is paramount to achieve a good control of food allergy and possibly to prevent further sensitization. Recent findings on the endotypes and ILRA variant add further support to the dual hypothesis of sensitization vs tolerance and pave the way for the use of biologicals active on the TH2 pathways of the allergic inflammation for reducing food allergy and atopic dermatitis. In conclusion, the ultimate goal is to change the natural history of food allergy and atopic dermatitis preventing the progress of the allergic disease i.e. the “Atopic March”.
Speakers: Prof. Dirk van Gysel, Prof. Klara Martinaskova and Prof. Regina Fölster-Holst
Report written by Dr. Nives Pustisek
Childhood viral exanthema: old and new
Prof. Dirk van Gysel (Belgium)
The definition and classification of exanthems has been presented in the introduction. Exanthems are acute mucocutaneous dermatoses with erythema (vasodilatation), urticaria (oedema, extravasation), papules (cellular infiltrate) and purpura (vessel wall damage). Exanthems can be viral (measelses, rubella, erythema infectiosum, exanthema subitem, varicella, enterovirus infection, adenovirus infection, EBV infection, HFM disease), bacterial (impetigo, SSSS, scarlet fever, meningococcal sepsis, Rocky Mountain spotted fever) and of various types (Gianotti- Crosti syndrome, Kawasaki syndrome, APEC, EEM – Stevens-Johnson syndrome). During the lecture, very good photos and case reports of old exanthems (classic presentation and atypical/new presentation) and new exanthems (due to novel viruses and due to population and vector movement) have been presented. For example, measles can be presented as classical measles, atypical measles, attenuated measles 6 days after vaccination and modified measles after 2 previous doses of MMR vaccine. Varicella is a very common viral exanthems caused by varicella zoster virus (HHV-3) and can be complicated as hemorrhagic varicella and bacterial superinfection. Herpes zoster is reactivation of varicella zoster virus and characterized with preeruptive, acute eruptive and chronic phase. Hutchinson’s sign in herpes zoster infection means skin lesions at the tip, the side and the root of the nose, representing the dermatomes of the external nasal and infratrochlear branches of the nasociliar nerve Hutchinson’s sign is associated with increased likelihood of ocular complications associated with infection. Ramsay Hunt syndrome (herpes zoster oticus) is reactivation of Varicella zoster virus involving the facial and auditory nerves. Exanthems with infectious mononucleosis can be in 3 patterns: maculopapular (in young child), morbililiform eruptions (associated with antibiotics, ampi or amoxi), and Gianotti-Crosti syndrome. The clinical presentations of Asymetric periflexural exanthema of childhood, associated skin manifestations with Epstein-Barr virus, Hand foot and mouth disease, Papular purpuric gloves and socks syndrome have been presented. Finally, the skin manifestation of COVID-19 (acute COVID 19, COVID-19 vaccine induced skin lesions) and travel-related diseases (imported dermatoses) have been presented.
In conclusion, different agents (viruses, drugs etc.) can cause a similar eruption and one single agent (virus, drugs etc.) can cause different eruptions. The clinical picture is often clear-cut. In some cases, a systematic approach is necessary to make the right diagnosis. Diagnostic approach in viral exanthems include history (age, season, drug intake, outbreaks, prodromal phase, fever, other complaints or symptoms, travel dates and destination), physical examination then laboratory and technical investigations.
Simultaneous infectious exanthems, a diagnostic challenge
Prof. Klara Martinaskova (Slovakia)
Exanthems in children are extremely common and its manifestations range from mild to severe diseases. An exanthem is defined as any eruptive skin rash that may be associated with lesions of the mucous membranes (enanthem), fever or others symptoms. Exanthem may be a manifestation of an infectious disease or an adverse reaction to drugs. Risk of severity of exanthema can be associated with primary or secondary immunodeficiency, genetic disorders, malignancy, immunopresive treatment, co-infection, post COVID syndrome. Co-infection happens when in some situation at the same time period we detect not only one pathogen. Some authors distinguish concomitant/co-infections and superinfections. Co-infection is occurring concurrently with the initial infection, while superinfections are those infections that follow on a previous infection. The cases of co-infections Hand foot and mouth disease with adenovirus, co- infections Parvovirus B19 and Mycopasma pneumonia, co-infection Enterovirosis B6, Mycoplasma pneumonie and Parvovirus B19 in imumunocomprimised 10-year old girl and others have been presented, as well as case reports of viral infections in COVID-19.
Paraviral exanthems
Prof. Regina Fölster-Holst (Germany)
Paraviral exanthem is a clinically distinctive exanthem. Viral infection is suspected but there is no direct virus-related cytopathogenic effect. Paraviral exanthem merely reflecting the immune response of the host. There are several paraviral exanthema, only a few of which have been presented, such as Gianotti-Crosti syndrome, Asymmetrical periflexural exanthem, Pityriasis rosea, Pityriasis lichenoides, Acute hemorrhacig edema of infancy, Papular purpuric gloves and socks syndrome (PPGS), Eruptive psudoangiomatosis, Eruptive hypomelanosis. The diagnostic criteria include morphology, distribution, history, general health, histology, blood test, swabs. Lots of microbes (viruses and bacteria) and vaccination (hepatitis B, measles-mumps-rubella vaccine) can be associated with Gianotti-Crosti syndrome. Viral reactivation and visceral infection are associated with Drug reaction with eosinophilia and systemic symptoms (DRESS). Viral antibody titer and virus-load correlate with the severity of DRESS. At the time of making the diagnosis, antibody titers can still be negative and the virus load without abnormalities. Virus diagnostic should be performed for the following viruses: HHV-6, HHV-7, CMV, EBV. Antiviral therapy is helpful, but only in combination with systemic corticosteroids. Very interesting case reports of paraviral exanthems have been presented, such as a case of infantile DRESS (Chow ML et al, 2018), Hemorrhagic Gianotti-Crosti syndrome in a one and half month-old infant (Sarma N et al, 2013), Gianotti-Crosti syndrome like reaction secondary to molluscum contagiosum (Estébanz A, 2020), Human herpes virus -6, -7 and Ebstein-Barr virus reactivation in pityriasis rosea during COVID-19 (2021), Unilateral mediothoracic exanthem (Chuh A et al, 2016). Different clinical presentations of parvovirus B19 infection have been presented: Erythema infectiosum (only cause), PPGS (can be cause by parvovirus B19, EBV, CMV, HHV6, Coxackie B6, Hepatitis B), Petechial exanthem in “Bathing Trunk distribution caused by Parvovirus B19 infection (Huerta-Brogeras M et al). Finally, very interesting newly described paraviral exanthems have been presented: Eruptive pseudangiomatosis association with echo- and adenovirus (Acta Derm Venerology, 2017), Eruptive angiomatosis triggered by COVID 19 vaccination (Shanshai et al, 2022) and Eruptive hypomelanosis (Eruption of hypopigmented maculae in siblings, Chuh, 2016; Eruptive hypomelanosis-first case reported outside Asia, Donne M et al, 2018).
At last, but not least, I would like to present an excellent lecture in the field of psychodermatology.
Speaker: Prof. Uwe Gieler
Report written by Dr. Nives Pustisek
Skin picking in youth – a problem for dermatologists?
Prof. Uwe Gieler (Germany)
Skin picking disorders can be explained as self-infliction with obsessive or impulsive behavior, skin symptoms are visible and evident. The patient is aware of their behavior and open to discussing it. There are no skin sensations (picking without symptoms). The patient has tried to change their behavior several times. Feelings of shame and disgust are present. Skin lesions are mostly localized on face, arms and head. Diagnostic criteria for excoriation (skin picking) disorder can be found in DSM-V (May 2013). Skin picking disorders include iatrogenic excoriation, onychophagia, onychotillomaina, trichotillomania, trichteriromania, nose picking and ear picking, cheek biting (Morcicatio buccarum), lip biting or picking, knuckle cracking behavior. It is important to distinguish skin picking form factitious disorder. Factitious disorder is most often kept secret, the patient will not tell us about the skin manipulation. The skin picking problem is not secret, you can always ask patients are they try to stop. Prevalence of skin picking is about 5,4%. Skin picking can be compulsive or impulsive. It is important to differentiate them because of different treatment options. When the skin picking is impulsive, it is important to clarify impulsive behavior (diary), skills and stopping impulsive picking whereas for obsessive-compulsive skin picking, one should clarify obsessive-compulsive behavior (diary), reduce obsessive picking positive empowerment. Some skin picking treatment skills are to occupy your hands with something else until the urge passes or put on gloves; when tempted to pick, care for your skin instead (for example, apply moisturizer); aim to hold off the urge for longer and longer each time if you can’t resist it altogether; keep skin clean to avoid infections; stay busy; get rid of tweezers, pins and any other instrument you might be tempted to use; try squeezing a soft ball or playing with blue tack instead. At the beginning of the treatment, speak about skin picking as an obsessive-compulsive or impulsive behavior and do not give advice such as just stop the behavior. Habit reversal techniques and several web sites for online therapy of skin picking have been presented.
Speaker: Prof. Rudolf Happle
Report written by Prof. Ivelina Yordanova
On May 20, 2022 in the heart of Bavaria in the cosmopolitan and charming city of Munich, after 2 years of pandemic, a remarkable event the 21st Congress of the European Association of Pediatric Dermatology was opened.
The event was officially opened by the President of the Association Prof. Veronica Kinsler and Prof. Andreas Wollenberg, Chairman of the Local Organizing Committee of the event in Munich.
The fantastic program of the event, which includes 6 plenary lectures and 16 thematic sessions, is scheduled to be presented by the world's leading experts in the world of pediatric dermatology.
The first of the six plenary lectures that opened the program of the scientific meeting was presented by the outstanding Prof. Rudolf Happle. He desided to start with his lecture on Mosaicism.
In his lecture Prof. Happle proposed a new category of cutaneous segmental mosaicism: isolated segmental biallelic monoclonal mosaicism. Prof. Happle said that different forms of cutaneous mosaicism have been delineated In autosomal dominant traits and characterized by multiple skin tumors such as neurofibromatosis 1, orcylindromatosis and other. The disseminated lesions originate from many different postzygotic events of loss of heterozygosity. In simple segmental mosaicism, a very early postzygotic new mutation gives rise to a segmental area that, due to different second hits, will later develop multiple biallelic tumors. In his lecture Prof. Happle draw attention to an unusual further category of segmental biallelic mosaicism that is related to autosomal dominant skin disorders in which the lesions develop as a consequence of two early mutations involving both alleles of a certain gene. This new category always occurs sporadically. Prof. Happle said that he and Prof.
Torello propose to call it ‘isolated segmental biallelic monoclonal mosaicsm’. A telling example is isolated large neurofibroma that is usually of the plexiform type. In general, sizeable tumors of this kind occur in patients with typical features of NF1, and are thus considered as a superimposed mosaic manifestation. Remarkably, however, there are many reports of large sporadic neurofibromas without any additional disseminated lesions of NF1, such as ordinary neurofibromas or café-au-lait macules or axillary freckling, and without any features of NF1 in family members. So far, the sporadic occurrence of such solitary large neurofibromas was difficult to explain. Prof. Happle said he propose that these cases may reflect isolated segmental monoclonal two-hit mosaicism. At an early developmental stage, a postzygotic NF1 mutation may quickly be followed by a second mutational event involving the same cell clone. It is important to realize that the second postzygotic mutational event, resulting in loss of the corresponding wild-type allele, should have occurred before or around the implantation of the blastocyst, because otherwise a segmental manifestation would no longer be possible, as can be concluded from analogous mosaic patterns reflecting X-inactivation. Prof. Happle emphasize the monoclonality of the second hit, which is in contrast to simple segmental mosaicism of biallelic skin disorders such as neurofibromatosis where the clinical lesions tend to originate from many different second hits and are thus derived from different cell clones. The new category should also be distinguished from simple segmental mosaicism of monoallelic skin disorders such as keratinopathic ichthyosis of Brocq and Siemens where the affected mutant cells carry one hit only.
Over and above, somatogonadal mosaicism cannot be excluded in the two other categories. Prof. Happle said these patient bears the risk to give birth to a child with full-blown keratinopathic Ichthyosis of Siemens, because of possibility of gonadosomatic mosaicism. He also presented two similar rare cases of segmental childhood-onset Hailey-Hailey disease and acantholytic epidermal naevus and palmoplantar kerathoderma, as examples for superimposed mosaicism.
Assoc. Prof. I. Yordanova and Prof. R. Happle
Prof. Rudolf Happle
Prof. Andreas Wollenberg
Speaker: Dr. Flora B. de Waard-van der Spek
Report written by Dr. Nives Pustisek
Dr. Flora B. de Waard-van der Spek is a Consultant in Pediatric Dermatology in Department of Dermatology, Erasmus University Medical Center Rotterdam and Kinder Haven Havenziekenhuis, Rotterdam, The Netherlands. She is a President of the EAACI Task Force Allergic Contact Dermatitis in Children and President of the Dutch Society for Pediatric Dermatology. Dr. de Waard-van der Spek said that allergic contact dermatitis (ACD) in children is increasing in the last years. It is very important to understand that ACD in young children is not rare, and should always be considered when children with recalcitrant eczema are encountered. he said that sensitization to contact allergens can start in early infancy, when the epidermal barrier is crucial for the development of sensitization and elicitation of ACD. Factors that may influence the onset of sensitization in children are atopic dermatitis, skin barrier defects and intense or repetitive contact with allergens. Allergic contact dermatitis because of haptens in shoes or shin guards should be considered in cases of persistent foot eruptions or sharply defined dermatitis on the lower legs. Clinical polymorphism of contact dermatitis to clothing may cause difficulties in diagnosing textile dermatitis. Toys are another potentially source of hapten exposure in children, especially from toycosmetic products such as perfumes, lipstick and eye shadow. The most frequent contact allergens in children are metals, fragrances, preservatives, neomycin, rubber chemicals and more recently also colourings.
She gave examples of allergic contact dermatitis in young children when their mothers using emollients containing bisabolol and other natural ingredients. She gave an example from her practice of developing allergic contact dermatitis to para-phenylenediamine, used in temporary tattoo dyes in children. She mentioned that as a result of these temporary tattoos, children develop long-term allergic contact dermatitis, which is why we, as doctors, recommend advising parents not to allow temporary tattoos on their children.
Dr. de Waard-van der Spek said that whenever, despite proper treatment of children with atopic dermatitis the rash persists, a patch test should always be done. Children should be patch-tested with a selection of allergens having the highest proportion of positive, relevant patch test reactions. The allergen exposure pattern differs between age groups and adolescents may also be exposed to occupational allergens.
In conclusion Dr. de Waard-van der Spek said, that in the diagnostic process of eczema in children, many products with which children have contact, may develop irritant or allergic contact dermatitis and should be considered.
Speakers: Prof. Judith Fischer and Dr. Juliette Mazereeuw-Hautier
Report written by Prof. Ivelina Yordanova
We attended an update on ichthyosis in a brilliant parallel session 3.
The session was chaired by Dr. Smail Hadj Rabia from the Department of Dermatology of the Hôpital Necker-Enfants Malades, Paris.
In this session Prof. Judith Fischer - medical director of Institute of Human Genetics, Albert-Ludwigs- Universität Freiburg Germany, presented the current classification and genetic features of the various forms of non-syndromic and syndromic ichthyosis. She emphasizes the phenotype and clinical features of individual diseases, the mode of inheritance and molecular genetic features, including affected genes and their function - germline mutations (in all body cells), mosaicism (postzygotic/somatic mutations) and combinations of the latter two.

Prof. Fischer presented 2 new syndromes from the group of syndromic ichthyosis. SAM syndrome is represented by congenital erythroderma with palmoplantar keratosis (striate, focal and diffuse), hypotrichosis and elevated serum immunoglobulin E. Both autosomal recessive inheritance (with biallelic loss of function mutation) and autosomal dominant inheritance with a heterozygous mutation in the transmembrane domain in DSG1 gene in SAM syndrome, have been established. In autosomal recessive SAM syndrome, desmoglein 1 deficiency leads to severe dermatitis, multiple allergies and metabolic disorders. Prof. Fischer showed a meta-analysis of mutations in the ALOX12B and ALOXE3 genes identified in a large cohort of 224 patients with ichthyosis in 2021. She emphasized the great importance of the lipid synthesis pathway in the stratum corneum and related mutations, as part protective function of the skin. In conclusion, Prof. Fischer emphasized that by 2022 more than 50 phenotypes of ichthyosis are known, 15% of the pathogenesis of which remains unclear. Genetic identification of unknown mutations through the new technology Whole-Genome-Sequencing WGS, which replace the old one WES, is expected. Prof. Fischer said that a new updated and integrative classification of ichthyosis is needed, which combines clinical and genetic features.
After this genetic lecture, Dr. Juliette Mazereeuw-Hautier, dermatologist from Paris focused on the main goals in the management of patients with ichthyosis - improving skin abnormalities, providing psycho-social support, training in therapy, genetic counseling and prevention of complications of the
eyes, ears, pain and itching, skin infections and delays in physical development, vitamin D deficiency, hair and nail abnormalities, excessive reactions to heat and cold. She presented the European Guideline for the Management of Congenital Ichthyosis, published in the British Journal of Dermatology in 2019, 180: 272-281. She emphasizes conventional treatment with emollients, keratolytics, topical and systemic steroids, moist wraps in the most severe cases. Dr Mazereeuw- Hautier also presented some new data from a double-blind, randomized controlled trial of patients with asymptomatic ichthyosis with oral vitamin D versus acitretin, published in the British Journal of Dermatology 2022. This study included 34 patients with various forms of non-syndromic ichthyosis.
No significant differences were found between the 2 arms of treatment at the end of the study in terms of the severity and quality of life of the patients, and no serious side effects were identified. By understanding the pathophysiology of ichthyosis, it is established that targeted anti-inflammatory treatment is also needed. Ichthyosis is no longer considered only a disease of keratinization, but also a disease in which the disrupted skin barrier induces a complex immune response similar to that of atopic dermatitis and psoriasis. The inclusion in the treatment of patients with ichthyosis of biological agents that inhibit key interleukins in atopic dermatitis and psoriasis are promising strategies in the treatment of ichthyosis. There are several publications from 2017 to the present that report that a
large number of ichthyosis has been found to have systemic T-cell activation and Th-17 / Th-22 polarization of patients' blood. There is a strong correlation between the increased genetic expression of the Th-17 pathway and the severity of ichthyosis and cutaneous erythema. Clinical trials are currently underway to treat adult patients with non-syndromic ichthyosis with Interleukin 17 - and Interleukin-12/23 inhibitors. The results are encouraging and the publication is a fact in February 2022 in the journal Archives of Dermatological Research. Gene therapy for ichthyosis is at a very early stage of development. There are two clinical trials in phase ½ for autosomal-recessive congenital ichthyosis (ARCI ichthyosis) due to a mutation in the gene responsible for the synthesis of transglutaminase 1 (TGM1) and Netherton's syndrome (SPINK5 gene). A new replacement therapy with a protease inhibitor, local recombinant alpha1-antitrypsin, has also been reported as a potential treatment for Netherton's syndrome.
Speaker: Dr. Talia Kakourou (Greece)
Report written by Dr. Rémi Maghia
Scabies is transmitted from person to person by direct contact; less frequently via contaminated objects. The parasite dies within 24 to 72 hours outside of a human host.
Animal scabies can only produce temporary eruptions in humans, since their parasites cannot survive in humans.
Prevalence in children is 5 to 10% in developing countries; it is unknown in Western countries.
Clinical manifestations of scabies are a type IV hypersensitivity reaction to the larva, its faeces and its eggs. Pruritus presents 2 to 6 weeks after a primary infestation, and 1 to 3 days after a re-infestation. A vesiculopapular exanthem may occur in children under 2.
Hyperkeratotic crusted scabies, also known as Norwegian scabies, is typically seen in elderly subjects, but it is sometimes observed in children in cases of primary or secondary immune deficiency. It is distinguished by an absence of pruritus, parasitic proliferation, and extremely high contagiousness.
A diagnosis of paediatric scabies is based on the following arguments. Pruritus that does not affect the head, except in newborns and very young children. The classic cutaneous signs and their topography. Direct examination of skin samples obtained by scraping Microscopy: 100% specificity, 40-90% sensitivity. Dermoscopy (flat delta sign marking the parasite’s head at the end of a burrow), videodermoscopy, confocal microscopy, OCT PCR (an article in PLoS Negl Trop Dis 2021).
The BJD 2020 provides consensus criteria for diagnosing scabies with a 3-level classification: A. confirmed scabies, B. clinical scabies, C. suspected scabies.
The differential diagnosis for newborns is: seborrhoeic dermatitis, nummular eczema, AD, infantile acropustulosis. For children: contact dermatitis, AD, viral exanthem, papular urticaria. For scabies nodules: mastocytomas, Langerhans cell histiocytosis.
Treating scabies: European recommendations, JEADV 2017
The recommended treatments are: permethrin cream 5%, oral ivermectin, benzyl benzoate lotion 10 to 25%.
Alternative treatments: malathion in aqueous solution 0.5%, ivermectin lotion 1%, sulphur ointment 6- 33%.
For crusted scabies: topical scabicide daily for 7 days, then 2 x/week and ivermectin on days 1, 2 and 8.
Permethrin is safe for use during pregnancy and breastfeeding; authorised for use in children starting at age 2. Sulphur ointment: authorised for newborns under 2 months. Benzyl benzoate: authorised during pregnancy, may cause skin irritation. Oral ivermectin is contraindicated for children weighing
less than 15 kg and in pregnant or breastfeeding women.
All members of the family must be treated at the same time.
The parasite is destroyed at temperatures above 50°. At the end of treatment, clothes and sheets must be washed at high temperature and ironed with a hot iron, or placed in a plastic bag for at least 3 days. Rugs and carpets must be vacuumed. Pesticide application is not indicated.
And here’s a surprising tip from the speaker! For unshakeable cases, she recommends leaving the house for a long weekend and doing the treatment. By the time the patients come back home, their house will be free of parasites!
Symptoms often persist for 2 weeks after a successful treatment: use medium-strength topical corticosteroids.
Children can go back to school the day after treatment. Classmates and teachers usually don’t need to be treated unless they show symptoms.
If symptoms persist after 3 or 4 weeks, consider: lack of compliance with treatment, re-infection, contact allergy to treatment, psychogenic pruritus, incorrect diagnosis.
Speaker: Prof. Jacob Mashiah (Tel Aviv, Israel)
Report written by Dr. Rémi Maghia
Ringworm is believed to be the most common fungal infection in children, especially for children aged 4 to 7. The fungistatic action of sebum would seem to explain its lower frequency of occurrence after puberty.
Ringworm is more common in boys than in girls, and in immunosuppressed patients as well.
The predominant species type varies by geographical location. For example, T. tonsurans and M. canis predominate in North America, versus M. canis, M. audouini, T. tonsurans, T. violaceum, T. mentagrophytes in Europe. The differences are explained by differences in habits, population movements (immigration, refugees), and locally available treatment options.
The diagnostic techniques are: direct examination, cultures, Wood’s lamp, trichoscopy, and PCR. Fungal culture is the standard with its high specificity and sensitivity, but this technique is slow.
Trichoscopy is a quick, inexpensive method with moderate overall sensitivity and specificity.
The specific trichoscopic signs of ringworm are: comma hairs, corkscrew hairs, Morse code hairs, zigzag hairs, block hairs and i-hairs. The prevalence, sensitivity, specificity, and positive and negative predictive value of all of these signs are well-known. The specificity of these particular signs is very high: 99-100%. It is also known which characteristics are associated with which species. For example, while corkscrew hairs are common to both microsporum and trichophyton species, trichophytic ringworm varieties do not typically present Morse code, zigzag or comma hairs.
We also know that during treatment, certain trichoscopic signs (not all) will have disappeared after 4 weeks, and that after 12 weeks, if the mycological exam is negative, the only remaining trichoscopic signs are perifollicular and/or diffuse desquamation.
“Healthy carriers” show dermatophyte colonisation without symptoms, and are a source of disease transmission. 32% of families with an infected child have at least one healthy carrier. This is especially the case for the anthropophilic species, with a minimal inflammatory response.
Treatments for ringworm include: griseofulvin, terbinafine, itraconazole and fluconazole. Griseofulvin has been the first-line treatment for 40 years. Sensitivity to this drug seems to be declining. Preferred option for microsporum infections.
Terbinafine is the only alternative to griseofulvin approved for ringworm by the FDA (not in France). Preferred option for trichophyton infections.
Itraconazole and fluconazole show increasing evidence of effectiveness, though perhaps less so for fluconazole, and require shorter treatments than with griseofulvin.
Topical treatments should only be considered as secondary treatments. Shampoos containing ketoconazole, selenium disulphide, or zinc pyrithione can be used to reduce systemic treatments or to treat healthy carriers.
Complementary measures
School attendance: a child with a combined treatment can return to school the next day, though some say a week when an anthropophilic species is involved.
Shaving the scalp: not required if topical treatment is used.
Family screening: systemic treatment if clinical symptoms are identified, antifungal shampoos in case of asymptomatic contact.
Decontamination of objects in the home (combs, toys, pets in case of a zoophilic species)
Speaker: Dr. Secil Vural (Turkey)
Report written by Dr. Rémi Maghia
Author’s note: There wasn’t really any paediatric angle to this presentation.
There are over 20 different species of Leishmania, including tropica, major, infantum, braziliensis, mexicana, guyanensis, panamensis.
12 million patients are infected worldwide, with over a million new patients every year from 100 endemic countries. Worldwide, 350 million people are at risk of infection, and as a result of climate change, the endemic zones are likely to expand due to the geographic expansion of sand flies.
The shared clinical presentation: first a papule at the site of the bite a few weeks or months later. Then spontaneous healing after 3 to 8 weeks, leaving an atrophic scar. The lesions occupy uncovered skin areas: centre of the face, extremities. In endemic zones, children are most commonly affected.
Development of CL: Acute CL can heal spontaneously with a scar, or without a scar when treated.
Chronic CL can develop into recurring CL in 2 to 5% of cases, into a anergic diffuse form (no healing) in 5 to 10% of cases. Or into lupoid CL (partial healing).
In cases of acute CL, the typical development of the lesion is an initial papule, then a painless nodule with a scab, then an ulceration, then healing with an atrophic scar. Healing time varies from 6 to 15 months for L. tropica to 2 to 6 months for L. major.
Recurring CL presents with new papular lesions during or after the healing process of an acute CL. The lesions may persist individually or coalesce over a period of several years.
Chronic CL: the initial lesions do not improve, persist without ulceration, and are resistant to treatment. There is a deficiency in cellular immunity. Clinically and histologically, it may resemble lupus vulgaris.
Diffuse CL: related to immunodepression, and may resemble lepromatous leprosy. Mucocutaneous leishmaniasis is caused by certain species (“L. braziliensis complex”). It is characterised by the destruction of the lips, palate, nasal septum and can result in death.
Diagnosis is performed by directly looking for the parasite in a skin sample taken from the border of the lesion (site of activity). PCR is currently the preferred diagnostic test: 54 to 99% sensitivity with a possibility of diagnosing the species. Culture in a special medium is also possible.
Treatments:
- Pentavalent antimonials, systemic or locally injected.
- Amphotericin B liposomal: effectiveness, no resistance observed, monitoring of renal and cardiac function. Expensive, unfortunately…
- Miltefosine: a promising drug for this indication. Low-level side effects: digestive effects, increased creatinine levels.
- Pentamidine
- Others: fluconazole, aminosidine, ketoconazole, itraconazole, terbinafine, azithromycin, zinc, rifampicin
- Cryotherapy: 1x/week, two cycles of freezing for 10 to 20 seconds, 2 mm of freezing of the healthy peripheral zone. High relapse rate. But combining with injections of antimonials increases effectiveness. Problems with scarring.
Monitoring therapeutic response: a 2/3 decrease in lesion size after 6 weeks is considered as a response to treatment, whereas a decrease of < 1/3 indicates changing to a different treatment. Post-treatment monitoring: every 3 months for a year to watch for possible recurrence. For L. braziliensis: after one year, annual follow-ups for 10 years.
Speaker: Dr. Ramon Grimalt (Spain)
Report written by Dr. Rémi Maghia
The most common nail anomalies in children are:
Before 2 years of age: onychoschizia and leukonychia. After 2 years of age: leukonychia and onychophagia.
Onychoschizia is the second most frequent nail anomaly. It involves splitting of the free edge of the distal nail plate into layers. This phenomenon is very commonly seen on the thumbs and great toes. While the aetiology is not precisely known, repeated trauma, frequent baths and use of solvents have been suggested as predisposing factors.
Leukonychia can be seen in the form of longitudinal white striations or white spots on fingernails or toenails. There are multiple causes.
Onychophagia
A distinction is made between onychophagia (nail biting) and onychotillomania (compulsive destruction of the nails), which are common in childhood. Incidence is 28 to 33% in children between 7 and 10 years old. Onychophagia should not be considered merely a cosmetic problem. A 2014 study that evaluated 450 children with a history of onychophagia showed that 75% of them had attention deficit and hyperactivity disorders.
Infections
Viral (warts, periungual and especially subungual, making treatment difficult), bacterial, fungal.
Systemic diseases
Capillaroscopy can be a great help: though not very specific, it can provide an early sign of systemic disease.
Genodermatoses
- Pure ectodermal dysplasia.
- Congenital pachyonychia.
- Congenital malalignment of the great toenails.
Terry’s nails
Diffuse leukonychia with “ground glass” appearance, described by Terry in 1954, associated with a diagnosis of hepatic cirrhosis in 82 out of 100 patients. Other associations were later described by Holzberg et al.: chronic congestive heart failure, diabetes. These authors also conclude that young patients with this nail anomaly have an increased risk of systemic disease. Recently, Fawcett linked Terry’s nails to hyperthyroidism.
Differential diagnosis of Terry’s nails includes comparison with Lindsay’s nails (half-and-half nails), associated with chronic kidney disease, and Muehrcke’s nails, associated with severe hypoalbuminaemia or in cases of antineoplastic drugs.
Traumas
Haemosiderin and melanin can have exactly the same appearance!
Psoriasis
These anomalies are numerous and well-known, and diagnosis is made easier if psoriasis lesions are detected elsewhere; in particular, remember to examine the scalp.
Alopecia areata
The prevalence of nail anomalies in children with alopecia areata was assessed at 46% (126/272) by Tosti et al. The start of nail symptoms may precede or follow the hair loss.
The nail changes are: very regular and superficial pitting, punctate leukonychia, trachyonychia/twenty- nail dystrophy: 12% of children (Tosti).
Speaker: Dr. Zsuzsanna Szalai (Hungary)
Report written by Dr. Rémi Maghia
With such a broad topic, it's impossible to be exhaustive. Here are a few selected excerpts, grouped by category.
I/ Skin as a sign of systemic disease
New eruptions
An unusual eruption, or one that does not respond to treatment or is accompanied by fever, joint or muscle pain, or other symptoms may indicate an internal problem or an infection. For example:
- Necrolytic acral erythema as a marker of hepatitis C.
- Dermatomyositis, which is a sign of internal cancer, e.g. ovarian cancer, in 20% of adult cases.
New “bulges”
- Eruptive xanthomas: may indicate elevated triglyceride levels, or may be a sign of uncontrolled diabetes.
- Eruptive xanthogranulomas: may reveal haematological disorders.
- Tumour progression.
Colour changes
- Yellowing of the skin may be a sign of liver disease.
- Darkening in folds or in exposed areas, joints, or old scars may be a sign of adrenal disease, such as Addison’s disease.
- Acanthosis nigricans can reveal the start of diabetes or be a sign of an internal cancerous tumour.
Changes in texture
Can be a sign of:
- Lymphoma
- Multiple myeloma
- Complement deficiency or amyloidosis.
II/ Skin and cancer
- Multiple eruptive seborrhoeic keratoses (sign of Leser-Trélat): may reveal an internal cancer (adenocarcinoma), but beware of overdiagnosis.
- Acute febrile neutrophilic dermatosis (Sweet’s syndrome), sometimes associated with haematologic cancer.
- Acquired ichthyosis or acquired pruritus which may indicate an occult cancer, often lymphoma.
- Paraneoplastic pemphigus: has been associated with various cancers, including leukaemias.
- Carcinoid syndrome: flushes and erythema on the neck in relation to a carcinoid tumour.
- Erythema gyratum repens: a very rare eruption associated with various cancers.
III/ Skin and endocrinopathy
- Hypo- or hyperthyroidism: may affect hair, nails and skin.
- Cushing syndrome: stretch marks, moon face, skin fragility.
- Addison’s disease: hyperpigmentation.
IV/ Skin signs of deficiencies
Periorificial and alopecia signs of acrodermatitis enteropathica are the iconic example, with a clear therapeutic test via zinc supplements which yield “dramatic” improvement of symptoms within a few days.
V/ Cutaneous signs of autoimmune diseases
The typical “raccoon-eye” appearance of facial erythema in neonatal lupus erythematosus
Speaker: Prof. Stephanie Christen-Zäch (Switzerland)
Report written by Dr. Rémi Maghia
Urticaria and angiooedema have numerous aetiologies: infectious, allergic, pseudo-allergic, physical, toxic, cholinergic, endogenous (autoimmune, autoinflammatory, endocrine, haematologic), and idiopathic.
If the angiooedema is isolated: C1 esterase deficiency, ACE use, hereditary bradykinin-mediated angiooedema.
Clinically, the classic form presents with areas of vasoconstriction around papular lesions, otherwise as shaped elements or dermographism, or as diffuse small papules in case of cholinergic urticaria. The particular form seen in babies is giant hives, which may have a haematic element such as ecchymoses, not to be confused with serum sickness. The hands and feet are often affected.
The following are not considered to be subtypes of urticaria: urticarial vasculitis (unchanging), serum sickness, acute haemorrhagic oedema of infancy, maculopapular cutaneous mastocytosis, autoinflammatory syndromes.
Distinctions are made between:
- Acute urticaria < 6 weeks: frequent in children, parainfectious, allergic or pseudo-allergic.
- Chronic urticaria > 6 weeks. Prevalence from 0.5% to 1.5% in children.
- Spontaneous urticaria: no triggering factor.
- Inducible urticaria: specific inducing factor.
The subtypes of chronic urticaria are:
- Spontaneous chronic urticaria (SCU), with known or unknown causes.
- Chronic inducible urticaria, triggered by different types of causes:
- Physical: cold, delayed reaction to pressure, heat, sun, dermographic, vibration
- Others: Aquagenic, cholinergic, contact
Duration of SCU: most commonly 1 to 5 years: 50% improve within 6 months, 20% within 3 years from the start.
The analysis does not need to be exhaustive and expensive: take the patient’s history and clinical exam into account. One exception: if there is a suspicion of type 1 food allergy or in the presence of triggering factors such as NSAIDs.
Pharmacological treatment of SCU
First line: Second-generation H1 blocker at standard dose
Continue for a few weeks after resolution, gradually decrease to determine whether maintenance treatment needs to be continued.
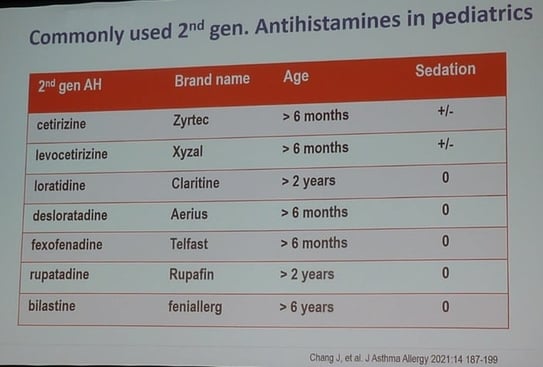
Second-generation H1 blocker used in paediatrics are: cetirizine, levocetirizine, loratadine, desloratadine, fexofenadine, rupatadine, bilastine.
Second line: if inadequately controlled after 2 to 4 weeks, or even earlier if symptoms are intolerable: increase dose up to x4.
Third line: add omalizumab, which is approved for children > 12 years old with CU, and for children with asthma from age 6.
Fourth line: if inadequate response at 6 weeks: cyclosporin, with the caveat that this treatment cannot be continued in light of the possible renal consequences.
Other therapeutic options: montelukast (leukotriene inhibitor), ranitidine (H2 blocker), doxepin (tricyclic antidepressant), systemic corticosteroids limited to 10 days for severe acute reactions; cease all use of aspirin and NSAIDs, which are exacerbating factors for CU
Speaker: Prof. Christine Bodemer (France)
Report written by Dr. Rémi Maghia
Mastocytoses result from an abnormal clonal accumulation of mast cells in at least one type of tissue (skin, digestive tract, liver, bone marrow, etc.). The release of mediators induces a mast cell activation syndrome (MCAS) consisting of urticaria, flushing, abdominal pain, diarrhoea, headache, bone pain, etc.
In children, spontaneous regression of the disease is typical, in contrast to adults in which there are non-regressive lesions and frequent systemic forms.
Somatic gain-of-function mutations are identified in 85% of paediatric mastocytoses. The D816V mutation of the KIT gene is found in only 42% of cases in children, in contrast to what is seen in adults (90%). The physiopathological role of this mutation is not yet entirely clear.
Sporadic forms (acquired mutations) are far more numerous than familial forms; less than 150 familial cases have been reported, of which only 13 had a germline KIT mutation (and never D816V).
The skin lesions specific to cutaneous mastocytosis (CM) are:
- Maculopapular CM (MPCM): can have large lesions or small lesions.
- Mastocytoma: nodular lesion.
- Diffuse CM (DCM).
MPCM (70 to 80%) occurs before the age of 2, and shows macular or papular lesions that are red/brown in colour (cf. “urticaria pigmentosa”). It is more or less diffuse, located mainly on the trunk and limbs. The scalp may be affected; in addition, the presence of blisters on the scalp at birth should point us to this diagnosis.
Mastoctyoma (10 to 20%): present mainly at birth or before 3 months. Brownish nodular lesion, most commonly just one and of variable size. There are frequent recurrences of blisters or superficial desquamation.
Diffuse CM (5%): neonatal occurrence, firm diffuse infiltration, bullous haemorrhagic detachments, “orange peel” appearance. Systemic risk, especially neonatal anaphylaxis.
Diagnosing paediatric CM
- Clinical signs. Positive Darier’s sign.
- Skin biopsy: very useful in unclear cases (Giemsa and c-KIT).
- Blood tryptase level (not systematically used for diagnosis). A high level is not always associated with the systemic forms, and is correlated with the skin surface involved.
- Check for c-KIT mutation? (This is not a prognostic factor for regression.)
Progression
- Regression is spontaneous in over 80% of cases, most often around the age of 6. Rarely seen: fatal progression or progression to an aggressive form and/or a sarcoma.
- Note that progression of MCAS reactions and regression of skin lesions do not always occur at the same time.
Treating paediatric CM
- Do not treat in all cases: important to reassure parents because this is a benign disease in over 99% of cases.
- Avoid factors that induce MCAS: physical factors, drugs (aspirin, codeine, morphine, NSAIDs).
- Diet only if justified.
- Anticipate anaphylaxis: in case of general anaesthesia (for the patient’s entire life).
- Provide a specific emergency card.
- If history of anaphylaxis or DCM: prescribe self-injectable adrenaline.
- Vaccinations: provide as normal if there has not been any serious anaphylaxis.
- Follow-up: every 6 months initially, then once a year. Monitor growth, presence of diarrhoea, flush, pruritus, dizzy spells…
- Tests (blood, imaging): depending on clinical signs.
Symptomatic treatments, in progressive lines:
- Topical corticosteroids (pruritus)
- H1 antagonists (pruritus)
- H2 antagonists: heartburn, diarrhoea, food reactions
- Combination of H1 and H2 antagonists
- Leukotriene receptor antagonist: montelukast
- Omalizumab
- Severe MCAS: combined treatments
In cases of diffuse blistering, systemic forms
- Corticosteroids: 0.5 to 1 mg/kg/day for a short period
- Imatinib in case of “paediatric” mutations
- Sirolimus in case of D816 mutation
Speaker: Dr. Anne-Sofie Halling (Denmark)
Report written by Dr. Rémi Maghia
The Danish patient register is known as a veritable treasure trove of raw patient data, and one that has given rise to numerous studies and articles in the medical and dermatological literature.
The Danish system includes multiple specific registers with data on the following areas: education, psychiatric illnesses, prescriptions, health care services, diabetes, cancer, causes of death, medical records of birth, income, and medical databases. The registers are interconnected, with a unique number assigned to each citizen across all of these public registers.
With regard to paediatrics, every Danish child up to age 18 is listed in the Danish National Patient
Registry. Statistics can be compiled by comparing the subjects being studied with controls matched to them by sex and age. The Cox regression model is used to calculate the hazard ratio.
The variables considered in the statistical models are age, sex, relevant comorbidities, medications, and socioeconomic status.
Danish study of comorbidities of atopic dermatitis (AD)
1) One out of every 4 children with AD has asthma (pooled OR: 2.78, 95% CI 2.5-3.1)
2) Children with AD have more severe asthma: we have figures for children with AD, and figures for children with AD + asthma, compared to the children in the general population, including those with asthma. Statistics were compiled on items like hospitalisations, asthma drugs, and use of corticosteroids, with significant ORs (between 1.3 and 2.58) and adjusted ORs (between 1.31 and 2.21).
3) Children with AD are more often hospitalised with extracutaneous infections: upper and lower respiratory tract, gastrointestinal, urinary tract, musculoskeletal, central nervous system, heart, and sepsis (all ORs are greater than 1). That being said, these figures need to be interpreted in detail, e.g. for the question of biotherapies or immunosuppressors that may promote infections.
4) The “psychiatric process” of children with AD
Here again, 14,283 children with AD were compared with 142,830 reference children. ORs were calculated on the basis of raw data, adjusted data, and data adjusted for atopic comorbidities. The items studied were: antidepressants, anxiolytics, centrally acting sympathomimetics, psychiatric consultations, and psychological consultations.
5) Drug prescriptions in children with AD
Here, a comparison of children with AD and adults with AD was performed for the following items: all topical corticosteroids combined, or separately for low, medium, high and very high potency topical corticosteroids and systemic corticosteroids. As a side note, we see in general that adults more frequently receive the highest-potency treatments.
6) Prescriptions: One out of three prescriptions was never picked up at the pharmacist’s!
7) Corticophobia in the parents of 3,437 children with AD:
In this case, a questionnaire was sent out via email. A high rate of corticophobia (TOPICOP score) was significantly associated with: low level of parental education, low total income, absence of AD in the mother, longer time between the first AD outbreak and the start of local corticosteroid therapy.
8) Corticophobia is real issue in the medical profession, and the score in that population is higher than with patients!
The highest corticophobia scores were found among pharmacists, then general practitioners, then paediatricians, then dermatologists